Gururaja, G. N.; Mobin, S. M.; Namboothiri, I. N. N. Formation of Five-Membered Cyclic Orthoesters from Tribromides with
Participation of a Neighboring Carbonyl Group Euro. J. Org. Chem. Early View Feb. 23rd 2011
So as promised, I will be updating more often now. So far this week has built on the success that was last week. I’ve been getting a lot of, surprisingly, successful reactions. And in good yield too, upwards of 70%! Plus, my new method continues to gain steam as more and more substrates seem to work under my reaction conditions. The suspense is killing me too, but I will certainly have to wait to tell you for the time being :P. Professor Tilley looks like he’s going to give a talk on some of the work we’ve done thus far (hopefully) at the next ACS meeting in Denver in the Fall. I may consider going to that one despite my high aversion to flying :/. Also, I will soon hear from the NSF GFP. Last year, I got honorable mention. While this was awesome, I really hope this year I get the actual award. I put about two months of work into it and so much editing it started to give me a headache! Getting that award isn’t really about the money to me. It’s really just the prestige associated with being selected while competing on a national level. But that’s enough on the personal side, let’s get to the chemistry!
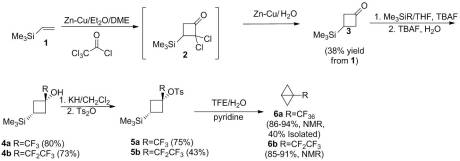
So I found this excellent article on my hiatus from blogging and I was attracted to it because of its unique but simple approach to the synthesis of previously unknown compounds. Moreover, it demonstrated the most important part of any organic synthesis: the workup. Some reactions work but if you cannot isolate the product, what’s the point? Purification is a biggie for me. If I plan on publishing something, I make damn sure that the compound I obtain is as pure as I can get it. And that’s one of the reasons I really enjoyed this article. They spent a good amount of effort on purification! Anyway the authors of this article (which is interesting dedicated to a legendary organic chemist, Ronald Breslow, who will be speaking at UConn in a few weeks) hoped to develop a novel method for the synthesis of orthoesters from relatively cheap starting materials. Moreover, they wanted to prepare synthetically valuable 2,2-dialkoxydihydrofuran which have limited known preparatory methods. I really liked flow of this article. The first thing the authors discuss is where this idea came from. It seems like it was off-shoot of some work that they were already doing with nitroalkenes. Essentially, they developed a selective method for the incorporation of methyltribromides or methylenedibromides using relatively simple Michael-like reactions:
Based on EPR data and some basic knowledge of the chemistry of organomagnesium compounds, Namboothiri and coworkers suggested that the magnesium-mediated reaction probably proceeded through a SET radical mechanism whereas the LDA conditions lead to an anionic mechanism. Either way, two equivalents of bromoform were required for successful Michael addition. From what it seems, they initially were just looking to expand the scope of their Mg-mediated reaction in this paper to include alpha, beta-unsaturated carbonyls. Seems like a logical step to me. That in it of itself could have been a short paper to Tet. Lett considering their high yields with a relatively broad scope. But the authors were quite clever here. They wanted to see what these sort of gamma-keto tribromides could be used for.
Their goal was to treat the tribromides with a basic ethanolic solution to see if they could transform the tribromidemethyl moiety into a carboxylic acid or ester functionality. However, they got more than they bargained for. By varying the workup of the crude reaction mixture, they found that they could either obtain a 2,2-dialkoxydihydrofuran (Neutral Workup) or a gamma-keto ester (Acidic Workup).
Namboothiri and coworkers, perceiving that the 2,2-dialkoxydihydrofuran was an intermediate in the formation of the gamma-keto ester, decided to optimize their conditions accordingly. They found that the optimal solvent for this reaction was a 1:1 mixture of EtOAc to EtOH as it homogenized the reaction mixture while providing excess ethanol. Upon optimization, they were able to conduct similar reactions on at least 7 other substrates. The only difficulty they encounter was when the aromatic rings in the tribromide were electron-withdrawing.
They then offered a particularly detailed mechanism accounting for the formation of the 2,2-dialkoxydihydrofuran under their conditions. The only issue I encountered is I was quite confused as to why an aromatic system would undergo room temperature addition of an alkoxide. My only guess is that it is such an electron-withdrawn (and highly delocalized) system, that addition by an anionic species has a low activation barrier. Plus the authors point out that it’s only stable under neutral workup conditions.
However, the authors didn’t stop there. They then optimized for the formation of the gamma-keto esters. The actual reaction conditions remained the same (solvent, time etc.) but the workup was adjusted. They found that they never obtained the lactone during this workup and that. by altering the alcoholic portion of the solvent, they could obtain a variety of esters and 2,2-dialkoxydihydrofuran. Moreover they found that they could take the 2,2-dialkoxydihydrofuran from their earlier optimization and hydrolyze them to give the gamma-keto esters in comparable yield. To put the nail in the coffin, they suggested a mechanism for this transformation:
This was a fine article by Namboothiri and co-workers and certainly worthy of EJOC (I even think they could of got a Org. Lett. out of it). I look forward to see more articles from their lab! I still have some more articles to review on the back burner, so stay turned! Ckellz…Signing off…