It’s been far too long since my last post. Between designing a total synthesis proposal for one of my classes, preparing a manuscript (and supporting information) for a project we are wrapping up, and (in the past week) doing a number of reactions, I’ve barely had time to read the literature, let alone blog. Moreover, I spent a week house hopping thanks to the surprise winter storm on Halloween that left most of Connecticut (myself including) without power for seven days. However, thanksgiving break has offered me a reprieve and I’ve managed to catch up on a lot that I was behind on. Chemistry continues to go well in the Leadbeater lab. Our collaboration with Dr. Tilley has picked up pace quite a bit with the successful isolation of another key substrate (which I was quite pleased about). We actually have been focusing on pushing out a continuous-flow project first now that it’s finally giving us promising results. I expect that a paper corresponding to that project should go out by next month at the latest. We are currently putting some finishing touches on another paper as well which should also go out by the end of next month (which I can’t wait to tell you all about!). Our paper with Dr. Fenteany’s group did not get into Nature Chemistry but we just submitted to Organic Letters so I’ll be sure to let you know how that process goes. I’ve also developed a new project based off some stuff I encountered while at Columbia that has allowed me to try my hand at a number of named reactions. I’m still just making a test substrate right now, but this one substrate offers a number of avenues to pursue. I’ve also been assisting another one of our lab members (DiAndra) with her project and she has been getting some very interesting (and exciting) results on what shaping up to be a pretty useful reaction. Other than that, Mike and myself have begun preparing lab experiments for the upcoming advance organic laboratory class we will be TAing for next semester. We hope to give the students experience with a broad range of reactions from transition-metal catalyzed couplings to organocatalysis. Our hope is that they will leave the course with the preparation they will need to begin graduate-level or industrial-level research.
In other news, I wanted to extend a warm congratulations to my friend Ryan Carris on his most recent publication (in JACS no less)! Ryan is a graduate student in the Johnson Lab at the University of South Carolina whom I met during my REU at Columbia. He’s doing some pretty good work down there, focusing on the manipulation of cyclopropanes for the construction of rather elaborate molecules. In his JACS communication, he details the umpoling of donor-acceptor cyclopropanes (in his case 2-vinylcyclopropane-1,1-dicarboxylate and related species) via a π-allyliridium complex. Carris and his fellow authors then exploit this umpole species to allow for alcohol and carbonyl allylations with a high degree of enantioselectivity (or diastereoselectivity depending on the substrate).They then showed the application of their research by making highly substituted lactones (which could serve as useful materials for a total synthesis) with excellent %ees (>90%). It’s pretty intereting work that’s well written so go check it out!
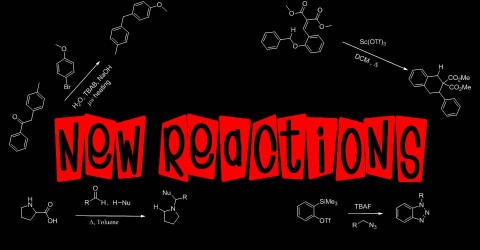
Now it’s time for something I haven’t done in ages, a review! I spent a lot of time catching up on articles in various journals (from Chemical Science to the European Journal of Organic Chemistry) but ultimately the most interesting article I found was in my favorite journal Organic Letters. About half a year ago I posted on some work that the Seidel group over at Rutgers was doing, regarding the exploitation of hydroxyproline decarboxylation for elaborate synthetic transformations. In this most recent article, Seidel and co-workers exploit the decarboxylation of proline itself (which to me is still somewhat of a weird concept) for a Strecker-like reaction.
The article begins with a brief overview of the Strecker reaction (which was discovered quite a long time ago in 1850). The article then diverges into specifics about decarboxylative reactions of proline along with some information about the resulting azomethine ylides (specifically their stability and reactivity).
Based on their previous experience with azomethine ylides, they rationalized that if they added a cyanide anion source to their reaction mixture, they could affect a Strecker-like transformations. Conceptually, what they wanted was imine formation between an aldehyde and proline, followed by a decarboxylation to give a azomethine ylide. This ylide could is in resonance with an alternative ylide. Protonation of that ylide followed by attack by cyanide at the iminium carbon would affect the first step in a Strecker-like synthesis.
To test whether this reaction was feasible, they looked into the reaction of proline with benzaldehyde in the presence of various cyanide sources under thermal conditions. In order to facilitate decarboxylation, high temperatures are required and their group found that microwave irradiation gave them the best results (especially since it allowed them to reach temperatures normally not readily accessible using conventional means). While many cyanide sources were tried, they ultimately found that TMS-CN gave them the best results. In fact, the reaction worked so well that it gave near quantitative yield.
With a relatively short optimization study behind them, they then proceeded to examine the scope of the reaction by first varying the starting aldehyde. A very broad range of aldehydes (from aryl to alkyl) were tolerated with only a few giving a mixture of regioisomers. Ketones proved far less reactive and lower yielding. After varying the carbonyl species, they then started altering the starting amino acid. They initially went with a previously successful unnatural amino acid, pipecolic acid. This amino acid gave solely the expected regioisomer as did tetrahydroisoquinoline-3-carboxylic acid. However when acyclic amino acid derivatives were used (N-benzyl glycine and N-methyl glycine), the unexpected regioisomer was the sole product. The authors attributed this to the substrate-level preferences for azomethine protonation.
During their study they noticed that if more proline was added, they could shift the preference for the unexpected regioisomer to the correct expected regioisomer. They suggested (in words and figures) the following mechanism to explain this trend:
This mechanism was supported some control experiments involving doping of proline or pipecolic acid in the presence of the unexpected regioisomers and conducting a reaction under their optimized conditions. They wrap-up the article with a small application. In a lesser known named reaction, the Bruylants reaction, α-cyano amines react with organometallic reagents (in particular Grignard reagents) in a substitution-like manner. This reaction relies on the concept that α-cyano amines are in at least a small equilibrium with their corresponding ion pair. Addition of the organometallic reagents leads to irreversible C-C bond formation thus driving the equilibrium towards substitution. Seidel and co-workers take the proline-derived α-cyano amine and react it with both an aryl and alkyl Grignard reagent to successfully give the corresponding α-alkyl/aryl amine.
Overall, this was an excellent article by Seidel and his students. Hats off for a job well done!! That’s all for now, I should be posting more regularly now, barring no more freak snow storms! Ckellz…Signing off…