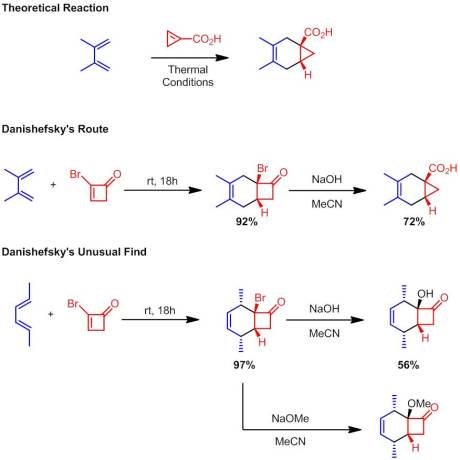
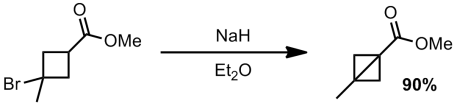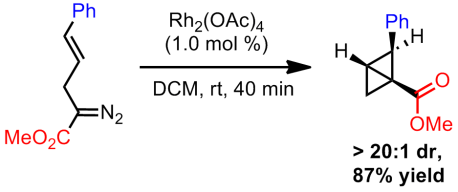Enantioselective Synthesis of 2-Arylbicyclo[1.1.0]butane Carboxylates Qin, C.; Davies, H. M. L. Org Lett. ASAP January 3rd, 2013
Happy New Year from New Reactions! 2013 brings another year of methodology blogging! It’s hard to believe that I started blogging two years ago this month. It most certainly has been one of my best decisions and I plan to continue doing it for a long time to come. After returning to lab this week, I have really begun cranking on some new projects in addition to finishing up two others. My hope would be to publish 3-4 research papers this year if possible. I just recently finished two review articles which should be published soon (one next month and one by the end of March). I’ll be sure to link them on NR once they are up. I am working closely with some of our undergrads as well (yes they do chemistry over winter break!) to hopefully get them on some papers. Additionally, we met with Professor Tilley yesterday as part of our collaboration with him (which is near completion!). I hope to send one of our excellent undergrads to work with him this summer.
Considering it has been little over a week since my last post (with a holiday there in between), I had a much smaller selection of new articles to choose from for this post. But in a way that was a good thing because I would have missed today’s article. While I do appreciate the need for chiral methodologies and high ees, they aren’t my cup of tea. I get excited for interesting, new transformations (especially those involving fluorine, but that’s beside the point). So I nearly skipped reading this new article from Davies at Emory because it started with “Enantioselective Synthesis of…”. However, the [1.1.0] managed to catch my attention. They are making bicyclobutanes! As you may already know, in addition to organofluorine chemistry, I loved strained systems. My undergrad research was focused on preparing bicyclobutanes so they always have a warm place in my heart. I was even more ecstatic when I found out that Davies cited my bicyclobutane paper!
For those of you who don’t know much about these systems, bicyclobutanes are some of the most strained systems known. The first bicyclobutyl system was prepared by Wiberg in 1959 via an anionic-ring closure:
Apparently they also were able to make the bromide tertiary and still perform the ring closure (although no synthesis of this bromide is given)
It wasn’t until a few years later (1963) that the unsubstituted system was prepared by Wiberg via a Wurtz coupling method:
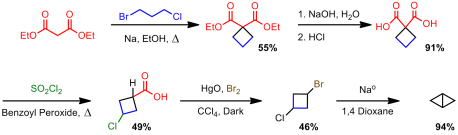
Finally, others started to get into the business of these small strained bicyclics. In the early 70s, Sieja, at du Pont, became very interested in synthesizing strained ring systems. You may remember his name from my last post. He was the first one to synthesize cyclobutenone. Not only that, but in a two year span from 1971 to 1972 he synthesized cyclobutanone as well as several bicyclobutane derivatives (Cyano, phenyl/polysubsituted/unsubstituted). His approach was, like Wiberg’s, anionic in nature. One route was to generate a carbanion via a Grignard process and do an internal displacement:
The other route was more akin to Wiberg’s 1959 work in which an acidic proton would be deprotonated to induce ring closure. In fact it was an expansion of the lesser known work by Blanchard:
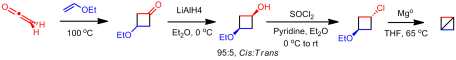
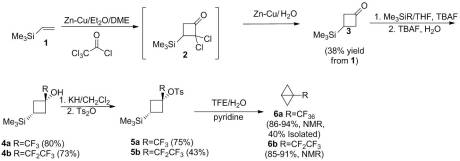
As far as I am aware, no additional work was done on preparing bicyclobutane or its derivatives until my article in 2011. Rather than using an anionic approaches, we investigated whether an cationic route could be used. Shiner showed that ring closure can be affected by 1,3 silyl elimination in the cyclohexyl manifold when the silyl group and the leaving group are in the ideal “cis” conformation. However, even in this ideal configuration, it is a minor product. We attempted a similar study in the cyclobutyl system. However reaching the desired sulfonate ester proved problematic as confirmed by Creary. We were able to pyrolzye it in situ to give low yield of the desired bicyclobutane. Unfortunately, this was by no means an efficient or effective method to synthesize the unsubstituted parent compound. Theorizing that the 1,3 elimination pathway could be enhanced by increasing electron demand at the cationic center, we installed a CF3 group at the -position. Isolation of the sulfonate ester was not only possible with this derivative but could be done in good yield. Solvolysis of this reactive species gave solely 1,3-silyl elimination, yielding the novel 1-(trifluoromethyl)bicyclo[1.1.0]butane. Similarly, the pentafluoroethyl derivative could be synthesized this way as well. The route I utilized is below:
Now that you know how it was made previously, how did Davies accomplish the synthesis of several bicyclobutyl derivatives? Well Davies, who for a long period of time was at the University of Buffalo, focus on enantioselective reactions utilizing chiral rhodium or dirhodium catalysts. He uses the carbenoid intermediates produced by these catalysts to perform cyclization reactions. While not solely his focus, he has used these catalysts to affect enantioselective cyclopropanation with remarkable success:
Seeking to further expand the scope of his methodology and develop the one of the first (if not the first) enantioselective route to bicyclobutanes, Davies explored a new system for cyclopropanation. He postulated that by simply extending the chain and placing the alkene within the same molecule as the putative carbenoid, he could obtain a double ring closing reaction:
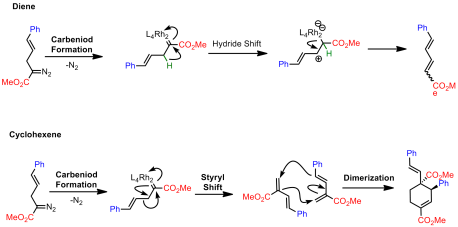
Using a simple model system, they explored Rh2(TPA)4 based on some reports by Fox indicating that this type of transformation would require a bulky rhodium species. However, rather than obtaining a bicyclobutane, a cyclohexene and a diene was instead isolated:
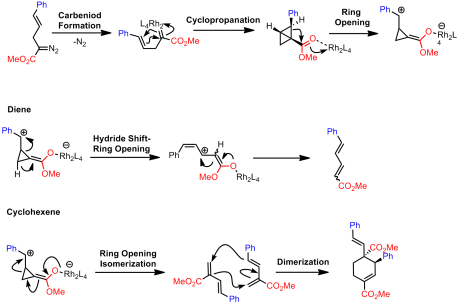
Davies argued that these product could result from two possible pathways. The first pathway involves no bicyclobutyl intermediates:
The second invokes the formation of bicyclobutyl derivatives. However, under the reaction conditions these strained systems further undergo reactions with the rhodium catalyst:
Both mechanisms are quite plausible. Later exploratory studies found that the bicyclobutanes could be obtained using Rh2(TPA)4 if ultra-low catalyst loadings were used in addition to short reaction times. However, both the cyclohexene and the diene were still being formed. Switching to Rh2(OAc)4, which is only slightly soluble in their reaction solvent (DCM), they were able to substantially improve selectivity towards bicyclobutane formation. Apparently this rhodium species does not catalyze the rearrangement to the diene/cyclohexene as readily and hence by using even shorter reaction times, strictly the bicyclobutane can be isolated. This catalyst gave excellent diastereoselectivity but no enantioselectivity.
Since Davies is interested in asymmetric transforms, chiral dirhodium catalysts were explored to facilitate an enantioselective cyclization. After some careful study, Rh2(R-BTPCP)4 was found to be the optimal catalyst. Using this catalyst, 9 bicylobutyl derivatives were prepared in moderate to excellent e.r. Davies did not forget about his cyclohexenes however. Using high loadings of Rh2(TPA)4 and longer reaction times, he showed that a variety of highly substituted cyclohexenes could be prepared.
Finally, with bicyclobutanes in hand, Davies sough to address the mechanistic question of which of the two pathways is operative. Using Rh2(TPA)4, at short reaction times (40 minutes), he showed that a mix of products could be obtained (diene/cyclohexene/bicyclobutane). By allowing the reaction mixture to stir for 12 hours, the remaining bicyclobutane starting material was completely converted into the expected products (mostly into the cyclohexene). He then proceeded to expose a previously isolated bicyclobutane to the same reaction conditions. After 40 minutes, a mixture of the diene/cyclohexene/bicyclobutane was obtained and after 12 hours no more bicyclobutane was detected. Rather it completely converted to the cyclohexene. However, since the ratios between the 40 minute two trials were drastically different, Davies concluded that both mechanism were occurring. He did state that, based on the ratios, direct conversion of the starting material to the cyclohexene is faster than decomposition of the product (which is in line with the necessity for short reaction times).
Well this certainly was an excellent article by Davies and I hope you all enjoyed it. It’s not too often you see a new route to bicyclobutanes, not to mention an enantioselective one! Hats off to the Davies group for an excellent job! That’s it for this week, Ckellz…signing off…