Eddy, N. A.; Kelly, C. B.; Mercadante, M. A.; Leadbeater, N. E.; Fenteany G. Access to Dienophilic Ene-Triketone Synthons by Oxidation of Diketones with an Oxoammonium Salt Org. Lett. ASAP December 29, 2011
Eddy, N. A.; Kelly, C. B.; Mercadante, M. A.; Leadbeater, N. E.; Fenteany G. Access to Dienophilic Ene-Triketone Synthons by Oxidation of Diketones with an Oxoammonium Salt Org. Lett. ASAP December 29, 2011
So that moment that you all have been waiting for (well lets be real, mostly the moment I have been waiting for) has arrived. I can finally share with you the project we worked on with Dr. Fenteany’s group this past year. After our group became interested in green oxidations using, surprise surprise, Bobbitt’s salt, we found that a graduate student upstairs had discovered something very unique. If 2,2 dimethyl-1,3-cyclohexadione was exposed to Bobbitt’s salt for an extended period, very little of the α-oxidation product was obtained. The student, Nick Eddy, was really interested in obtaining this another project he was working.
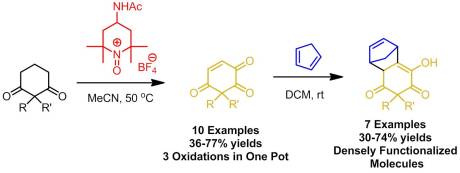
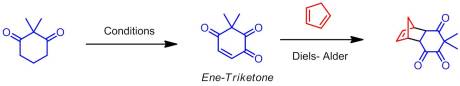
However, he noticed that another unusual product was obtained because some of the starting material had been consumed. After careful NMR analysis, he determined that the product was in fact an overly oxidized derivative of his starting material, an ene-triketone. Now at that point, it was fundamentally interesting but was it useful? After doing a bit of reading, he soon found out that the compound he has prepared normally took four steps to make and at 15% overall yield. No other ene-triketone had been reported in the literature, likely because of the difficulty of their synthesis. After talking with us about it, we agreed that if we could optimize the reaction to give a high yield of the ene-triketone and turn this into a project, it would likely be of high interest to the organic community. So we spent some time optimizing the reaction, ultimately finding that reaction only occurred at slightly elevated temperatures in highly polar solvents (DMF, MeCN). Moreover, it required a large loading of salt (3.8 equiv)! On a large scale, this meant greater than 60 grams of salt. So we became experts at preparing Bobbitt’s salt (which, as I’ve said, is very easy).
We also found that the quality of the salt needed to the be the highest it possibly could be. It needed to be absolutely free from impurities and water (as did the solvent). We needed to not only recrystallize the salt from boiling water but also rigorously dry it using not a desiccator but with a Abderhalden over KOH and ethanol! This boosted our yield from 20-30% using the normal powder version of the salt to 60-80% using the highly purified salt. With that accomplished, we then prepared a range of 2,2 disubstituted cyclohexadiones. Now you may wonder why it was necessary to block that 2 position (in between the two ketones). That is to prevent enolization at that position (very stable enol) and oxidation to give the 1,2,3 triketone which has been reported. Therefore we tried a variety of substituents with mixed results. Most of the ones we tried worked (and the syntheses of these substrates were amazingly fun)! We also got some meaningful mechanistic information out of the substrates we tried. The following two results:

told us that the driving force of the reaction was that first ene-like reaction and sterics (which was said to be, in their system, disfavorable by the AZADO article I just reviewed) and subsequent oxidation to the triketone. From there, the triketone immediately enolizes and reacts again. Since there is a acid proton in the vicinity, it eliminates to generate the olefin of the ene-triketone. We need for excess salt, heating, and the polarity of the solvent all make sense with the mechanism (stabilizing enol form, 3 oxidations, steric repulsion). The mechanism is by far the best part of the article to me so here it is in all its glory:
At that point we could have stopped and submitted the work. But we weren’t satisfied with just the oxidation, we wanted to see what sort of reactions we could do with the ene-triketones (being the only people to have access to them at that time). We decided that an excellent application would be as dienophiles in Diels-Alder reactions. That olefin is very electron-withdrawn and therefore would likely react in a shot. And, as predicted it did at room temperature! We found that most of our ene-triketones reacted quite well with fair to excellent endo : exo ratios. The sheer complexity of the molecules were generating was what really impressed me though. Well I really hope you go take a look at our article for more details and enjoy it! It certainly made my day yesterday! Ckellz…Signing Off…