Ye, S.; Wu, J. Org. Lett. ASAP Oct 20th 2011
With work coming back to a (slightly) more regular pace, I now actually have to blog on a more regular even (gasp) weekly basis! Not all too much new to report this week. We are pretty much at a standstill with all our pending publications. Even our research has been, at best, coming along slowly. I did manage to have some late-day success Friday synthesizing a particularly hard compound to make. While the yield wasn’t the best, I’m going to retry it and scale it up because it is somewhat critical to our project with Dr. Tilley. Meanwhile, I’ve come up with a couple of new ideas to try, in addition to searching for a molecule to synthesize (and developing an appropriate synthesis). Mike has been doing the same. We also have the exciting (and somewhat daunting) task of designing an undergraduate advanced organic chemistry lab. The goal of this course is to give the undergrads the tools necessary to begin conducting research and prepare them for graduate school. We want to teach how to run flash columns (and learn other more advanced forms of purification), get them to perform some more complicated reactions (and we are open to suggestions!) as well as teaching them how to write scientifically. Moreover, it’s useful for us in that we will be learning how to design a course (and for someone looking at academia like me, such a skill is quite useful). I still haven’t decided where exactly I’ll end up (industry or academia) but I will likely carry out a post-doc after I complete my Ph.D. at UConn. I’ve even had a few thoughts as to where I would like to go for my post-doc. But that’s a ways off; I still have my thesis, classes, teaching and all sorts of other things to get done first. On an unrelated note, I’d like to mention I’ve added quite a number of links (thanks to a very useful post on BRSM) I hope you find them as useful as I and some of my lab mates have! And now, on to the lit!
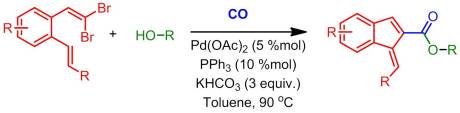
This weeks article is from, surprise surprise, Org. Lett. As promised the focus of this article isn’t fluorine. Rather, we have some useful and mechanistically interesting palladium chemistry developed by the Wu group. The article begins with a discussion of the history of palladium chemistry, in particular with respect to carbonylation. If you don’t already know, carbon monoxide is a very useful material for the functionalization of aryl halides under mild conditions. I’ve in fact conducted such reactions and, for the most part, they are pretty reliable. With the recent rise in popularity of gem-dihaloolefins in the literature, it was simply a matter of time before these substrates were explored for carbonylation procedures. In fact, Wu had just recently explored it for use in Suzuki coupling reactions (forming indenes). Not surprisingly, indenes are quite useful in material science and chemical biology, so more routes to access these sorts of structures is beneficial. Instead of simply continuing on with their Suzuki work, Wu and co-workers attempted to prepare functionalized indenes (e.g. 1-methylene-1H-indene-2-carboxylates) via carbonylation. They theorized that 1,2 disubstituted aryl systems (in which one substituent was a gem-dihaloolefin and the other was some sort of cross coupling partner such as another point of unsaturation) could undergo a tandem Heck-carbonylation reaction.
Starting with (E)-methyl 3-(2-(2,2-dibromovinyl)phenyl)acrylate (which is surprisingly easy to prepare), Wu and co-workers conducted an extensive optimization study, ultimately finding that the ideal solvent was toluene, and most effective catalyst was palladium(II) acetate with PPh3 added in as a ligand. As one would expect, the carbonylated intermediate was trapped with an alcohol (in their optimization study, n-butanol). They found that the ideal base was in fact KHCO3.
Once optimized, they then screened pretty much every alcohol under the sun, from trifluoroethanol to naphthol (to me this didn’t prove all too much, just that you could make different esters, but it did get them more substrates). They then switched over to the more important variable, the gem-dibromoolefin. Initially, they had little to no luck until they realized that 4 angstrom molecular sieves were a necessary additive in order to achieve adequate yields. Unfortunately this limited them to phenols (likely due to competitive dehydration reactions). Therefore, they used 3-methylphenol as their “alcohol” source. With these modifications in place, Wu and co-workers decided to switch the substitution pattern on the ring up a bit (both EWGs and EDGs were tolerated under their conditions). Next, they changed the substituent on the other olefin. While changing it to a ketone or phenyl ring proved unamendable to their reaction, esters modifications and nitriles were well- tolerated. Overall the yields on the reactions that worked were good and they provided a reasonable mechanism.
While I did like this article, I felt it wasn’t all that impactful. I was displeased with the fact that more of their substrates were simple alcohol swaps. However, the transformation (tandem heck-carbonylation) was very cool in itself so congratulations to the Wu group for a pretty good article. That’s all for this week. Ckellz…Signing off…